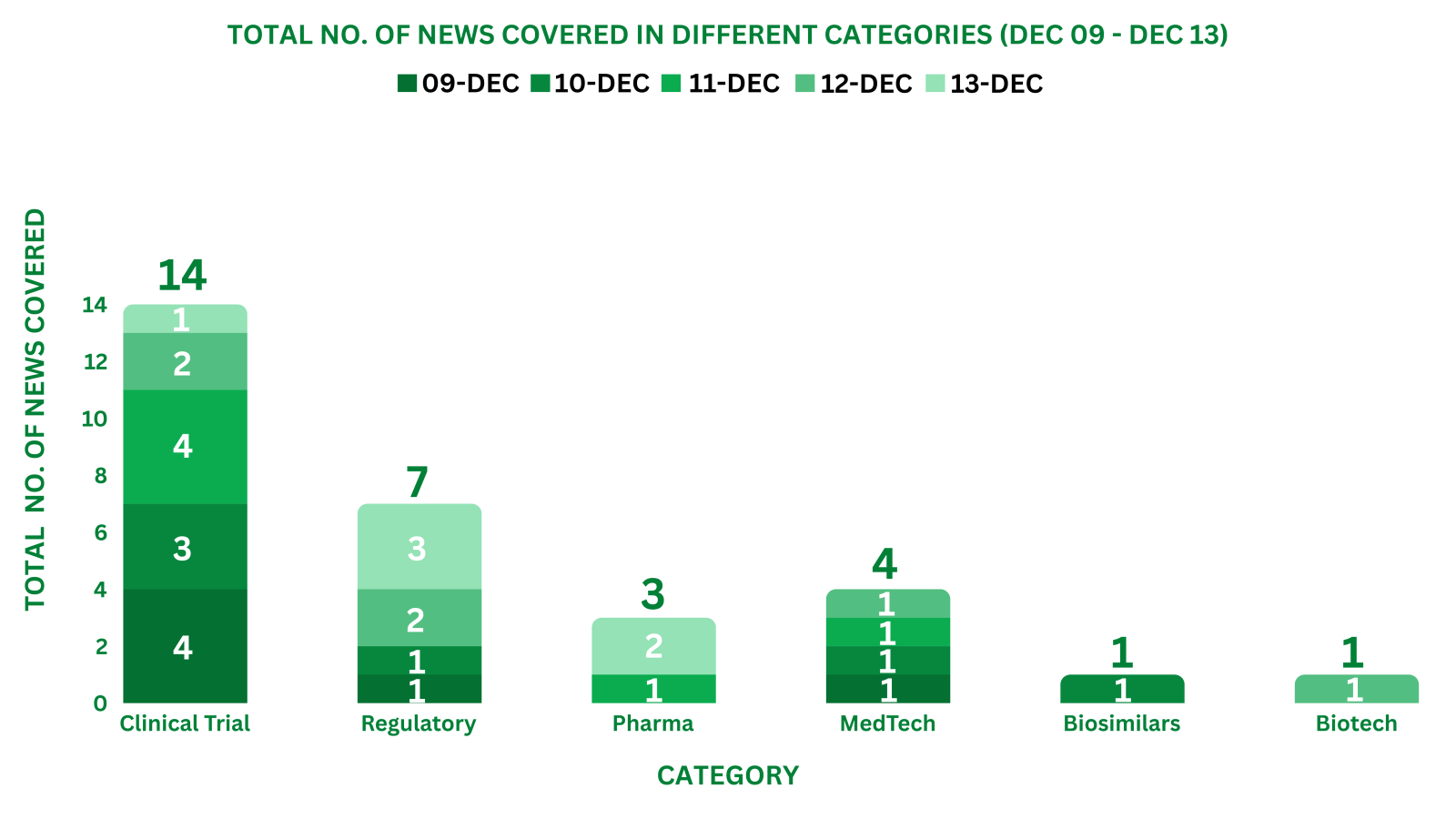
PharmaShots Weekly Snapshots (December 09 – December 13, 2024)
This week PharmaShots’ news was all about the updates on Clinical Trials, Regulatory, Pharma, MedTech, Biosimilars & Biotech. Check out our full report below:

AbbVie and Genmab Highlight P-I/II (EPCORE NHL-2) Study Data of Epcoritamab to Treat R/R Follicular Lymphoma (FL) at ASH 2024
Read More: AbbVie and Genmab
Merck Highlights the P-II (WaveLINE-007) Study Data of Zilovertamab Vedotin to Treat Diffuse Large B-Cell Lymphoma at ASH 2024
Read More: Merck
AstraZeneca to Feature Data from P-III (AMPLIFY) Study of Calquence Plus Venetoclax to Treat Chronic Lymphocytic Leukaemia at ASH 2024
Read More: AstraZeneca
Roche Highlights 5-year Analysis from P-III (POLARIX) Study of Polivy (Polatuzumab Vedotin) for Diffuse Large B-Cell Lymphoma (DLBCL) at ASH 2024
Read More: Roche
AbbVie Reports Results from the P-III (TEMPO-2) Study of Tavapadon for Parkinson's Disease
Read More: AbbVie
Merck Reports the P-III (KEYLYNK-001) Study Data of Keytruda for Treating Advanced Epithelial Ovarian Cancer
Read More: Merck
Eli Lilly to Highlight P-III (BRUIN CLL-321) Trial Data of Jaypirca (Pirtobrutinib) for Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma at ASH 2024
Read More: Eli Lilly
Incyte Highlights the P-III (inMIND) Study Data of Monjuvi (Tafasitamab) to Treat R/R Follicular Lymphoma at ASH 2024
Read More: Incyte
Novartis Reports Longer-Term Results from P-III (NATALEE) Trial of Kisqali (Ribociclib) to Treat Early Breast Cancer
Read More: Novartis
Daiichi Sankyo Begins the P-III (QuANTUM-Wild) Study of Vanflyta (Quizartinib) to Treat FLT3-ITD Negative Acute Myeloid Leukemia (AML)
Read More: Daiichi Sankyo
Dizal Reports Pooled Analysis from the Trials of Sunvozertinib to Treat Non-Small Cell Lung Cancer
Read More: Dizal
Eli Lilly Reports the P-III (EMBER-3) Trial Results of Imlunestrant to Treat ER+/HER2- Advanced Breast Cancer
Read More: Eli Lilly
AstraZeneca and Merck Report P-III (OlympiA) Study of Lynparza to Treat Early Breast Cancer
Read More: AstraZeneca and Merck
Repare Therapeutics Reports the P-I (MYTHIC) Trial Data of Lunresertib Plus Camonsertib to Treat Gynecologic Cancers
Read More: Repare Therapeutics

GSK Reports the US FDA’s Acceptance of Nucala (Mepolizumab) as an Add-on Treatment for COPD
Read More: GSK
Seres Therapeutics’ SER-155 Secures the US FDA’s Breakthrough Therapy Designation to Reduce Bloodstream Infections
Read More: Seres Therapeutics
HUTCHMED’s Orpathys and Tagrisso Combination Secures the NMPA’s Breakthrough Therapy Designation for Lung Cancer
Read More: HUTCHMED
SpliceBio Receives the US FDA IND Clearance to Initiate P-I/II Trial of SB-007 for Treating Stargardt Disease
Read More: SpliceBio
Sobi Reports the US FDA’s sNDA Acceptance of Doptelet (Avatrombopag) to Treat Pediatric Immune Thrombocytopenia
Read More: Sobi
Roche Reports the EMA’s Approval of Vabysmo Prefilled Syringe (PFS) for three Retinal Conditions Causing Blindness
Read More: Roche
Ascendis Pharma Reports the US FDA’s sBLA Acceptance of TransCon hGH for Treating Growth Hormone Deficiency
Read More: Ascendis Pharma
Relation Partners with GSK to Develop Therapies Targeting Fibrotic Diseases and Osteoarthritis
Read More: Relation and GSK
BeiGene and CSPC Pharmaceutical Collaborate to Develop SYH2039 for Solid Tumors
Read More: BeiGene and CSPC Pharmaceutical
HUTCHMED Secures $10M Milestone Payment from Takeda Following the First Reimbursement of Fruzaqla in Europe
Read More: HUTCHMED

AngioDynamics’ NanoKnife System Secures the US FDA’s 510(k) Clearance for Prostate Tissue Ablation
Read More: AngioDynamics
SetPoint Medical Reports PMA Submission of SetPoint System to the US FDA for Treating Rheumatoid Arthritis
Read More: SetPoint Medical
Microbot Medical Reports Regulatory Submission for LIBERTY Endovascular Robotic System to the US FDA
Read More: Microbot Medical
RapidPulse Secures the US FDA’s Approval to Initiate IDE Study of Cyclic Aspiration System in Patients with Acute Ischemic Stroke
Read More: RapidPulse

Celltrion’s Omlyclo (CT-P39) Secures the Health Canada’s Approval (Biosimilar, Xolair)
Read More: Celltrion

KIND Pharmaceutical Reports Preclinical Results of AND017 in Animal Models of Multiple Disorders
Read More: KIND Pharmaceutical
Related Post: PharmaShots Weekly Snapshots (December 02 – December 06, 2024)
Tags

Disha is a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.